Repackaged Avastin® Service
Repackaged Avastin (bevacizumab) from Leiters Health
FDA-Compliant. Low Particulate. Ready-to-Use.
Our commitment to quality, compliance, physician satisfaction, and patient safety includes:
Meeting the FDA Guidance for Repackaging of Biologics.
Utilizing the StaClear® syringe.
Performing robust testing and providing the testing results via a Certificate of Analysis with every shipment.
Leiters Health recently sat down with Dr. Tanya Ghosh, an ophthalmologist from the Munnerlynn Eye Institute. Dr. Ghosh shared her passion for ophthalmology and the importance of high-quality repackaged Avastin for her patients and her retina practice.
Leiters Health is committed to meeting the 2018 FDA Guidance on Mixing, Diluting and Repackaging Biologics Outside the Scope of an Approved Biologics License Application1
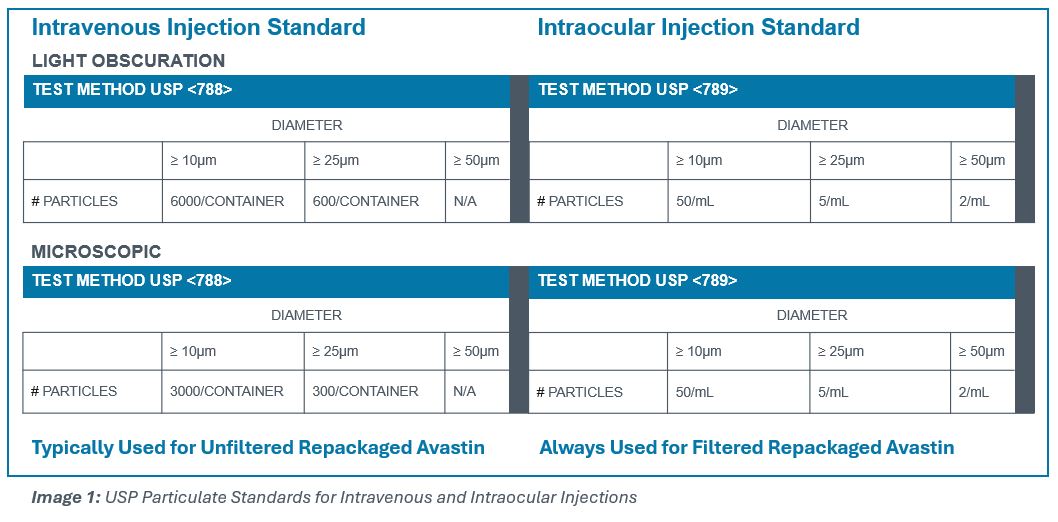
Avastin was neither developed nor manufactured to be an ophthalmic drug. Not surprisingly, according to Hoffmann-La Roche, bevacizumab is manufactured to meet the requirements for particulate matter in injections, USP <788>2,not the more stringent requirements for particulate matter in ophthalmic solutions under USP <789>. As a result, in repackaged Avastin, silicon oil microdroplets and protein aggregates have been a recurring issue3.
Leiters Health was the first 503B outsourcer to introduce and provide repackaged Avastin (bevacizumab) in accordance with the FDA’s 2018 Final Guidance for the Repackaging of Biologics. To achieve the low-particulate standards imposed by USP <789>, Leiters Health utilizes an optimized aseptic process combined with an advanced filtration to remove silicon oil microdroplets and protein aggregates from the Avastin.
To be compliant, repackaging of Avastin must comply with USP <789>. The Pharmacopoeial standards for the manufacture of intravitreal injections are different from those for intravenous administration with respect to the amounts of subvisible particles permitted. The USP manufacturing requirements for intravenous drug formulations (USP <788>) permit higher subvisible particulate counts than those for ophthalmic solutions (<USP 789>), by a significant magnitude. See Image 1.
Read this Industry Bulletin to learn more about the FDA’s Final Guidance for the Repackaging of Biologics and our journey and commitment to meeting the guidance.
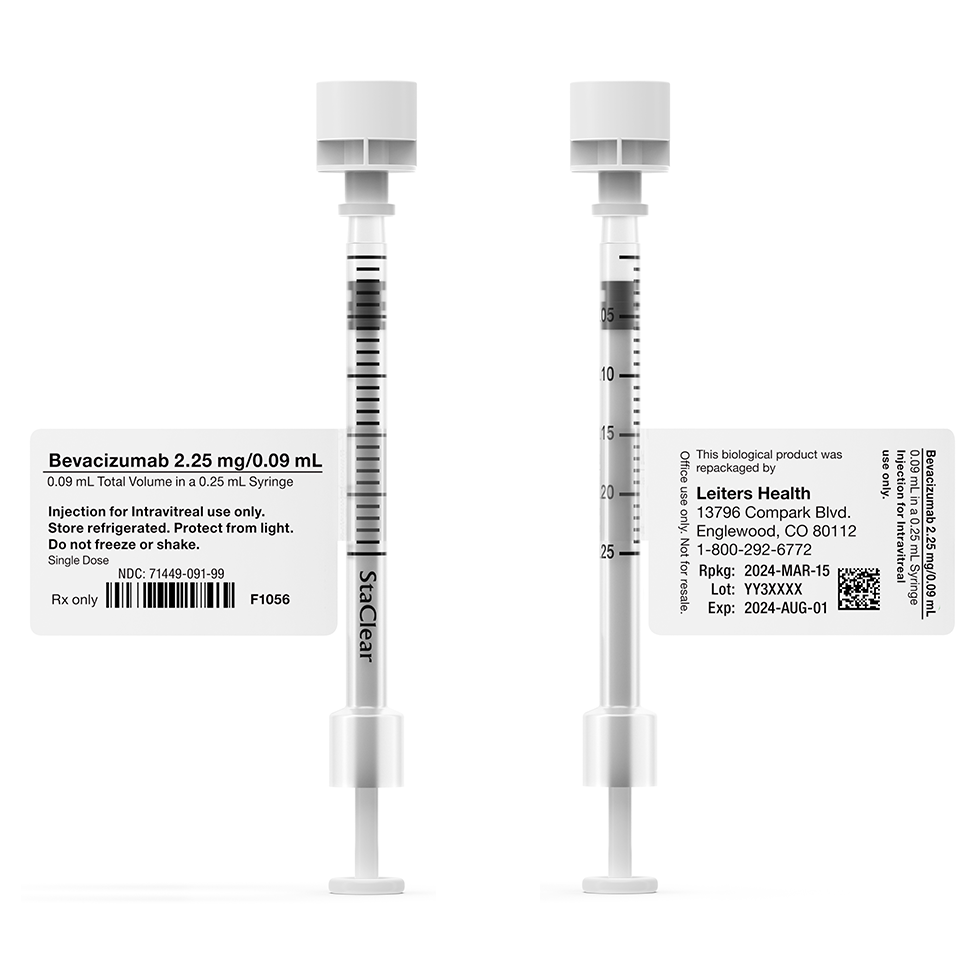
Choosing the right syringe is critically important to meeting the low USP <789> particulate standards required by the FDA Guidance on Repackaging Biologics.
Most syringes require free-floating slip agents such as silicone or oleamide to provide for easy and smooth plunger movement. These slip agents can mix with the solution in the syringe to increase particulates4. To prevent this from happening, Leiters Health syringe of choice for repackaged Avastin is the StaClear syringe.

StaClear Syringe Features and Benefits6
- Meets the FDAs chemical and biocompatibility standards for ophthalmic use.
- Polydimethylsiloxane slip agent is crosslinked and fixed onto the syringe barrel surface, creating an ultra-low particulate.
- Easy-to-read graduation marks.
Removable Butterfly Syringe Labels Now Available!
We are excited to unveil a removeable butterfly syringe label for our repackaged Avastin syringe products. These labels were developed in response to feedback from our retina specialists requesting a removable label that provides improved handling/use of the StaClear syringe during injection procedures.
Enhanced Clarity, Readability, and Compliance with Regulatory Standards
Our labels have been meticulously redesigned to ensure all vital information is easily readable. All repackaged Avastin syringes are clearly labeled, meeting Industry label standards for the Drug Quality and Security Act (DQSA), USP General Chapter 7 Labeling, and the FDA’s 2018 Final Guidance for the Repackaging of Biologics. Labeling includes appropriately sized and spaced lettering, presentation specifications, barcodes, repackaging dating, lot number, expiration dating, and storage requirements.
Improved Usability
Our labels are perforated for easy removal to avoid label interference with and/or handling during injections.
The Leiters Health Convenience Fill Provides Physicians With An Educated Needle Choice
Leiters Health recognizes that a physician’s choice of syringe and injection needles has an impact on dosing accuracy due to the retained fluid in the needle/syringe combination. Due to variable fluid retention (i.e. dead space), the volume of Avastin that is delivered to the patient depends on the combination of syringe, fill volume, and needle selection.
To better inform our customers, Leiters Health polled retina customers to determine the most used needles for intravitreal injections and conducted extensive internal testing of these needles with Leiters Health’s repackaged Avastin and the StaClear syringe. To download the results, please complete the form.
To receive a copy of the needle data showing expressed Avastin quantities, please provide the following information and a member of our team will be in touch shortly.
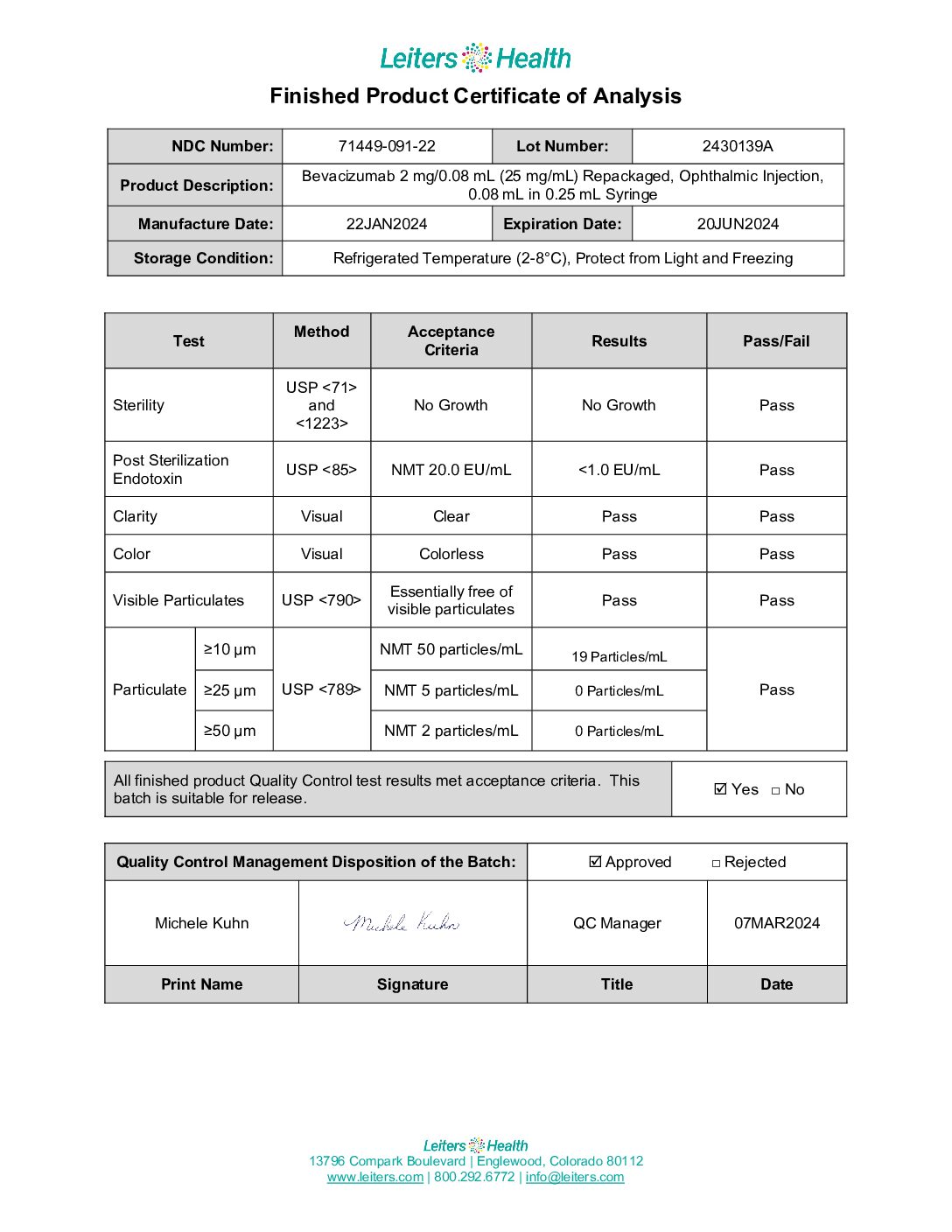
Leiters Health performs robust testing and reports all repackaged Avastin USP <789> testing results on a Certificate of Analysis (CoA).
This means you are receiving verified testing results for every syringe you receive. Not all repackagers of Avastin perform this level of testing or provide this level of detail.
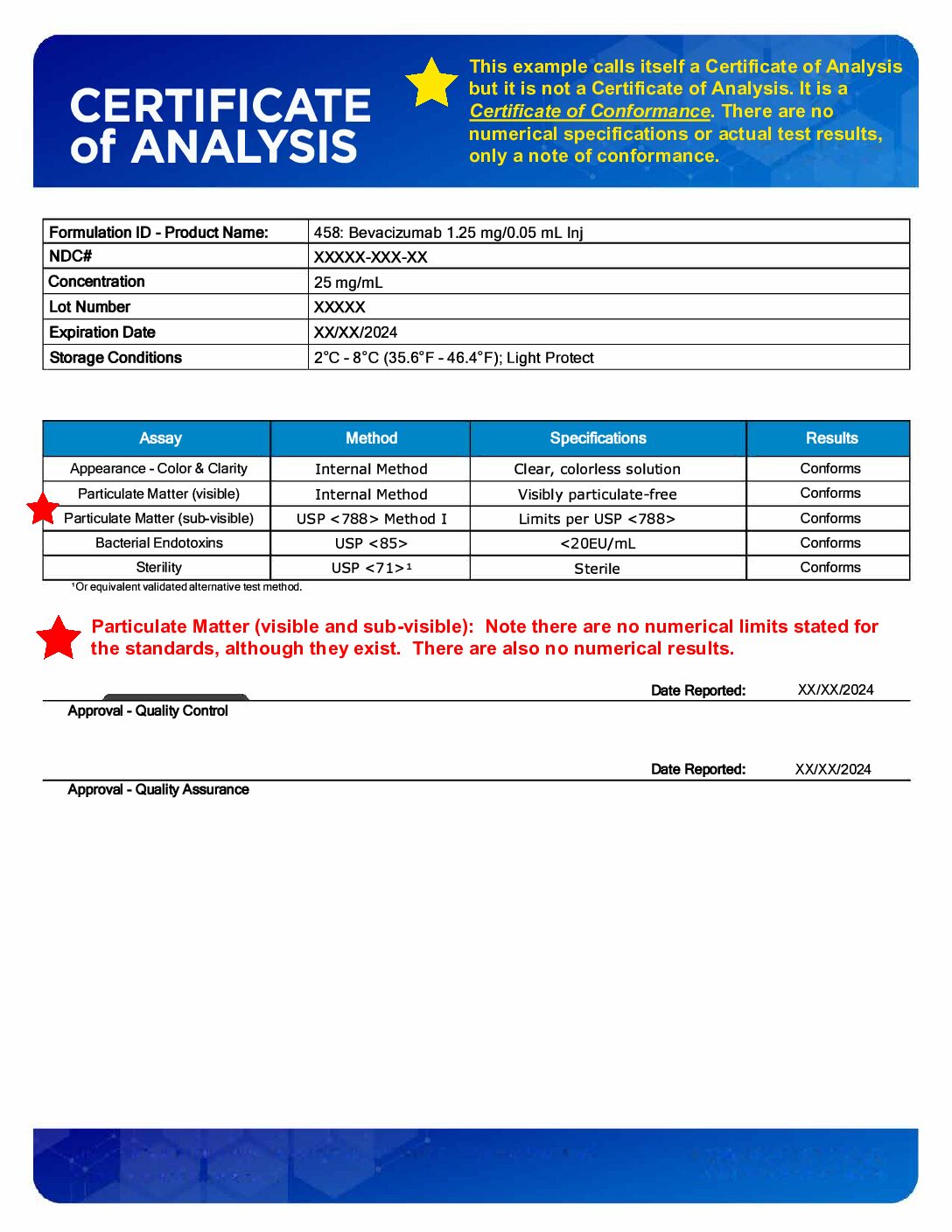
A Certificate of Analysis (CoA) provides detailed test results and analysis of a specific batch of product, verifying it meets predefined specifications.
A Certificate of Conformance (CoC) certifies that a product meets specified standards and requirements, without necessarily including detailed test data.
The main difference is that a CoA includes specific quantitative data, while a CoC is a declaration of conformity.
Because the required testing in the 2018 FDA Guidance on Mixing, Diluting and Repackaging Biologics requires significant product testing, especially particulate testing under USP <789> (the Intraocular injection standard), Leiters Health produces and provides a CoA for every lot of repackaged Avastin (Image 2).
Some 503B repackagers of Avastin who do not conform to the 2018 FDA Guidance on Mixing, Diluting and Repackaging Biologics test only to USP <788> (the Intravenous injection standard) produce only a CoC. A CoC does not provide testing data for each lot (Image 3).
Image 2: Leiters Health Certificate of Analysis (CoA)
Image 3: Example Certificate of Conformance (CoC)
The Leiters’ Difference Makes a Difference for your Patients
- We source Avastin so you don’t have to.
- We utilize an advanced filtration process prior to repackaging the Avastin per FDA Guidelines which results in reduced particulates.
- A Certificate of Analysis (CoA) detailing the testing is provided with every shipment.
- USP <789> compliant for visible and subvisible particulates.
- 150-day beyond use date (BUD)7.
- Compact space-saving tray to maximize refrigeration space at your facility.
- Each tray comes with 10 each pre-filled ready-to-use syringes.
Ready to place an order?
Reliability with Reserved Inventory
Leiters Health sources Avastin so you don’t have to. We streamline and simplify your supply chain by securing the inventory you need to flex with your practice demand, and can allow your staff to focus on patient care rather than managing two supply chains.
Benefits include:
- We produce and maintain a 30-day inventory of repackaged Avastin syringes so you can flex up and down your supply with your patient schedule.
- Same day shipping for orders received by 2pm MST.
Increased Access and Convenience with Leiters Online Ordering Portal
The Leiters Online Ordering Portal (LOOP) is an interactive, online tool providing a convenient way to order and manage the medications you purchase from us.
Benefits include:
- Convenient online ordering 24 hours a day / 7 days a week.
- Access detailed account and product information.
- Establish automated subscriptions and ordering templates.
- Learn more about LOOP
Footnotes/References
Avastin® is a registered trademark of Genentech, Inc.
StaClear® is a registered trademark owned by TriboFilm Research, Inc.
1 FDA Guidance Document: Mixing, Diluting, or Repackaging Biological Products Outside the Scope of an Approved Biologics License Application. Guidance for Industry; Jan. 2018 https://www.fda.gov/regulatory-information/search-fda-guidance-documents/mixing-diluting-or-repackaging-biological-products-outside-scope-approved-biologics-license
2 Hoffmann-La Roche Ltd., F. (2016). Application for the deletion of bevacizumab (Avastin®) on the WHO Model List of Essential Medicines. 21st Expert Committee on the Selection and Use of Essential Medicines, 1-6.
3 Effects of Long term Storage and Product Mishandling, Univ. of Colorado; Feb. 2011
4 CONTAMINATION OF ANTI-VEGF DRUGS FOR INTRAVITREAL INJECTION: How Do Repackaging and Newly Developed Syringes Affect the Amount of Silicone Oil Droplets and Protein Aggregates? Oct. 2018 https://pubmed.ncbi.nlm.nih.gov/28841584/
7 BUD is from date compounded or repackaged.